In a new paper, “Forest Resources of the World: Present Status and Future Prospects,” Singh et al. affirm the importance of forests for terrestrial biodiversity, provision of multiple ecosystem services, and supporting the economic well-being of approximately 1.6 billion people directly. This equals about a quarter of Earth’s population. The authors conclude that achieving global Sustainable Development Goals (SDGs), including poverty reduction, food security, and mitigating and adapting to climate change — all depend on sustaining forests.
According to the 2020 Global Forest Resource Assessment, Earth’s forested area comprises ~4.06 billion hectares, or 31% of the total land surface.More than half (54%) of all global forest area is found in five countries: the Russian Federation, Brazil, Canada, the United States, and China. Tropical forests constitute 45% of this total; boreal forests, 27%; temperate forests, 16%; and subtropical forests, 11%. An estimated 93% (3.75 billion ha) regenerate through natural processes; 7% (290 million ha) is planted forest.
The extent of global forest area has been declining for decades but the rate of loss slowed significantly between 1990 and 2020. This reflects decreased deforestation in some countries and an increase in forest area in others. The latter is due to both afforestation and also natural forest growth. However, conversion of tropical forests to agriculture continues apace. From 2010 to 2020, the net loss of forest area was highest in Africa (3.9 million ha) and South America (2.6 million ha). Increases in net forest area occurred in Asia, Oceania and Europe. The status of the top 10 countries or territories in global forest resources as of 2020 is given in Table 1.2 of the chapter. [News sources document that rapid deforestation continues in Brazil, at least.]
Several trends are concerning to those of us who value primary or undisturbed forests. First, the area of naturally regenerating forest has decreased, while the area of planted forest has expanded – but only by 123 million ha. In the last decade, the rate of increase in the area of planted forests has also slowed.
Second, total carbon stock in forests declined from 668 gigatons to 662 gt in 1990–2020. This is only 6%, but it is trending in the wrong direction. As we know, forest conservation counters climate change in two ways: conserved forests are a carbon sink, while degraded or destroyed forests are a significant source of atmospheric CO2. In fact, forests are the 2nd largest storehouses of carbon, after oceans. Global forests sequester about one-third of total CO2 emission from the combustion of fossil fuels. Almost all forest carbon is found in living biomass (44%) and soil organic matter (45%).
Third, primary forests are already severely reduced and continue to shrink. Primary forests are those composed of native species, and supporting relatively undisturbed ecological processes. They are irreplaceable for sustaining biological diversity. These forests are already severely reduced – they cover only ~ 1 billion ha. Since 1990, the extent of primary forest has decreased by 81 million ha. More than half are in Brazil, Canada, and Russia.
Singh et al. report that only about 10% of the world’s forests are set aside for biodiversity conservation. Again, trends are in the wrong direction. The rate of increase in the area of forest designated largely for biodiversity conservation has slowed. On the other hand, forest areas designated for other non-extractive purposes have increased: soil and water conservation, recreation, tourism, education, research, and the protection of cultural and spiritual sites.
Singh et al. are cheered by the fact that more than 2 billion hectares are under management with well-defined management plans. The extent of forests under management plans has increased by 233 million ha since 2000.
Singh et al. say that continuously increasing anthropogenic pressure is the main cause of deforestation and forest degradation in unmanaged forests. Citing projections that the world’s population will reach almost 10 billion by 2050, they say this growth will make reconciling the need for forest conservation with the basic requirements of humans for food, shelter, and fuel more difficult than ever.
I appreciate this honesty. Too many experts interviewed on the day that the global population was estimated at 8 billion made optimistic statements about the consequences. They mentioned Earth’s carrying capacity only in reference to First World people demanding excessive resources. There was minimal discussion of humanity’s carbon footprint and no reference to ever-increasing threats to biological diversity. Nor to the fact that people in developing countries want to raise their standards of living – which entails higher demand for resources, including energy. For an example, see The Washington Post editorial, here.
On the other hand, Ruby Mellen in the Post on 15 November mentioned that, according to the World Wildlife Fund, 75% of Earth’s ice-free land has been significantly altered by people, and two-thirds of mammal, fish, reptile, and amphibian species have become endangered in the last ~50 years. Unfortunately, the on-line version of the paper doesn’t have this specific article!
Threats to Forests: Fire
Singh et al. rank fire as the most disastrous threat, affecting biodiversity and carbon sequestration potential. According to the U.N. Food and Agriculture Organization, about 29% of the total geographical area in the world was affected by forest fires during 2001–2018; more than two-thirds of these fires occurred in Africa. U.S. media, however, focused on fires in the Amazon, temperate areas (U.S., Europe), and, sometimes, boreal forests or Australia. Singh et al. say that areas that are frequently affected by fire are prone to other types of disturbances like drought and outbreaks of insect pests.

Threats to Forests: Diseases and Pests
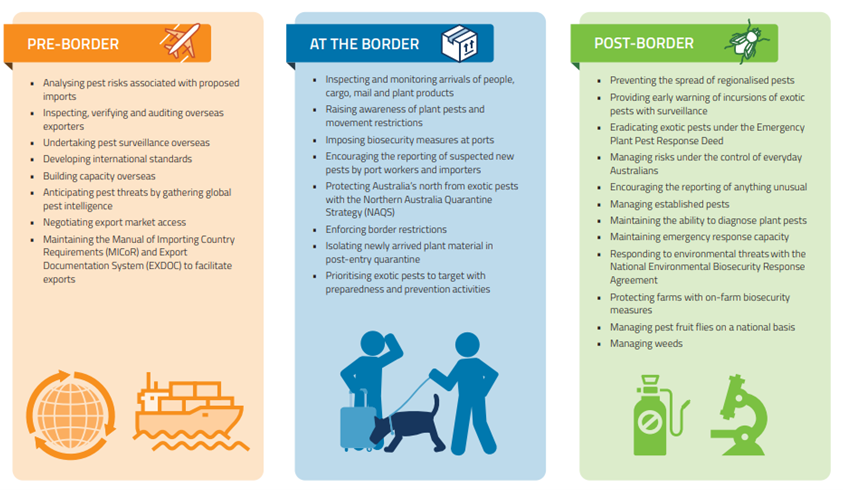
I am glad that Singh et al. recognize the damage to forest productivity caused by disease and pest infestations. In doing so, they cite familiar sources – Clive Brasier, Peter Vitousek, Juliann Aukema, Gary Lovett, Sandy Liebhold, Kerry Britton, Bitty Roy, Hanno Seebens – regarding surges in pest attacks; the growing diversity of damaging pests; resulting changes in forest species composition and structure that impede ecosystem functions and productivity. Singh et al. follow these sources in calling for improved hygiene in nurseries, adoption of scientific silvicultural practices reducing physical damage to the vegetation, selection of genotypes that are resistant, and reinforcing national and international policies on quarantine and biosecurity measures to minimize pest impacts in the future. They also mention adoption of remote sensing technologies to detect the trees under stress and use of sentinel plantings. They list the 10 most important international agreements dealing with invasive species issues as the International Plant Protection Convention, Ramsar Convention, Convention on International Trade in Endangered Species of Wild Fauna and Flora, Convention on Migratory Species, Convention on Biological Diversity and its Cartagena Protocol on Biosafety, IUCN Invasive Species Specialist Group, World Trade Organization Agreement on Sanitary and Phytosanitary Measures, Global Invasive Species Program, and International Civil Aviation Organization, and Cartagena.
Threats to forests: Development Projects
Singh et al. consider development projects to be the third threat to forest conservation. Their roads, powerlines, and other linear developments cause habitat loss and fragment landscapes. In their view, environmental impact assessments and other similar requirements are not yet sufficient to safeguard sustainable use of forest resources.
Policy Responses
Singh et al. call for more inclusive forest management structures to respond to the threat climate change poses to forests, industries, and forest-dependent communities. They all for partnerships that bring together researchers from several disciplines with forest managers and local stakeholders. Geoffrey M. Williams and others (including me) advocate for similar conservation approaches. (See pre-print here.)
In this context, Singh et al. mention several reports, plans, and agreements aimed at global forest conservation. Participants in global fora have recognized the importance of forests in contributing to food security and sustainable development. Among agreements mentioned are the UN’s Strategic Plan for Forests 2030 and recommendations of the International Institute for Sustainable Development (IISD) published in 1994. The former tries to generate greater coherence, collaboration, and synergy across UN programs aimed at encouraging volunteer forest conservation by countries, international, regional, and local organizations, partners, and stakeholders. Unfortunately, they do not discuss the extent to which the 30-year old IISD recommendations have – or have not – been implemented.
They also describe Forest Landscape Restoration as an effective strategy to restore the functionality of forests.Again, the focus is on a collaborative approach aimed at integrating efforts by all forestry-related stakeholders, e.g., scientific and academic organizations, local communities, indigenous peoples, and private sectors, including forest-based enterprises and NGOs.
Also praised is rising attention to trees outside forest. This includes fostering use of trees in agroforestry systems ranging from home gardens to farm forestry systems, shelterbelts, and woodlots. This approach helps to sustain the livelihoods of rural communities and maintain a stable and secure food supply. Meanwhile, it reduces dependence on natural forests
Singh et al. say community forest management and decentralized governance have gained acceptance. They describe examples from Gambia and Rwanda. They concede that such decentralization has its own risks and challenges. For example, e the most marginalized sections of the community must be ensured adequate capacity for robust conflict resolution.
Singh et al. advocate that all nations seek to increase their forest cover; affluent countries that are hampered by physical and climatic conditions should aid poorer nations in increasing and upgrading their forest cover. They suggest “recognition” and encouragement of countries that maintain forest cover above 30% of territory.
See also about loss of floral diversity and blog about IUCN’s global forest assessment.
SOURCE
Singh, M., N.N. Shahina, S. Das, A. Arshad, S. Siril, D. Barman, U. Mog, P. Panwar, G. Shukla, and S. Chakravarty. 2022. Forest Resources of the World: Present Status and Future Prospects. In Panwar, P., G. Shukla, J.A. Bhat, S. Chakravarty. 2022. Editors. Land Degradation Neutrality: Achieving SDG 15 by Forest Management; ISBN 978-981-19-5477-1 ISBN 978-981-19-5478-8 (eBook)
https://doi.org/10.1007/978-981-19-5478-8
Posted by Faith Campbell
We welcome comments that supplement or correct factual information, suggest new approaches, or promote thoughtful consideration. We post comments that disagree with us — but not those we judge to be not civil or inflammatory.
For a detailed discussion of the policies and practices that have allowed these pests to enter and spread – and that do not promote effective restoration strategies – review the Fading Forests report at http://treeimprovement.utk.edu/FadingForests.htm
or